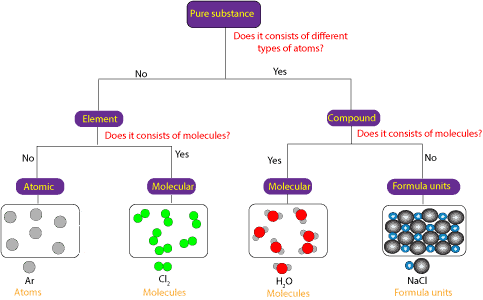
Is a compound a pure substance
Is A Compound A Pure Substance. Chemical compounds are pure substances which contain two or more chemical elements. A compound is a pure substance because its molecule cannot be broken down into simpler particles by physical means. A pure substance basically composed of two or more elements and chemically combined in a fixed proportion is called a compound. Properties of a compound.
Matter Pure Substances Vs Mixtures Review From docs.google.com
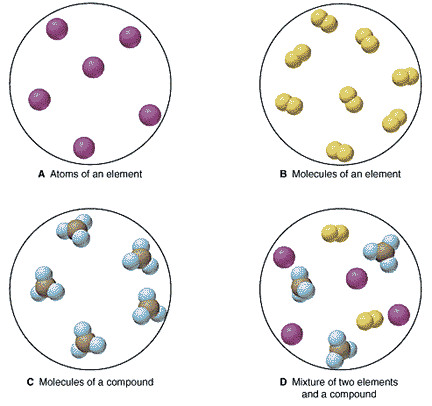
A compound is not a pure substance because it is not an element and only elements are pure substances. It has two elements hydrogen and oxygen combined in a fixed ratio. However in many ways the designation pure compound is an oxymoron since all compounds are pure. Matter can be divided into pure substance and mixture then pure substance can be divided into element and compound so from this classification we can say that a compound is a pure substance in pure substance the composition remains fixed an element is formed by only one type of atoms or by atoms which have same atomic number on the other hand a compound is formed by more than one element. Chemical compounds are pure substances which contain two or more chemical elements. Therefore water is a compound.
They are held together in a specific pattern by chemical bonds.
A substance is one of two forms of matter the other is a mixture. Properties of a compound. Chemical compounds are pure substances which contain two or more chemical elements. A substance is one of two forms of matter the other is a mixture. Therefore water is a compound. However in many ways the designation pure compound is an oxymoron since all compounds are pure.
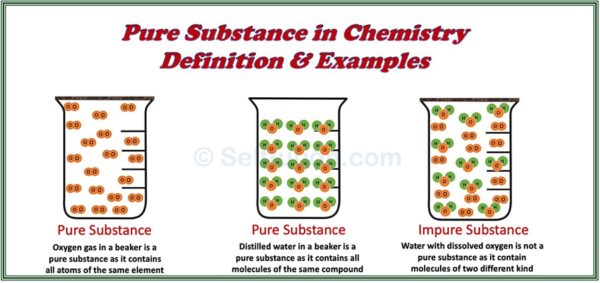 Source: selftution.com
Source: selftution.com
However in many ways the designation pure compound is an oxymoron since all compounds are pure. A compound is a pure substance because it consists of two different elements which are pure substances. However in many ways the designation pure compound is an oxymoron since all compounds are pure. A substance is one of two forms of matter the other is a mixture. A compound is not a pure substance because it is not an element and only elements are pure substances.
Source: chemteam.info
A substance is one of two forms of matter the other is a mixture. To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. It has two elements hydrogen and oxygen combined in a fixed ratio. However in many ways the designation pure compound is an oxymoron since all compounds are pure. A compound is not a pure substance because it is not an element and only elements are pure substances.
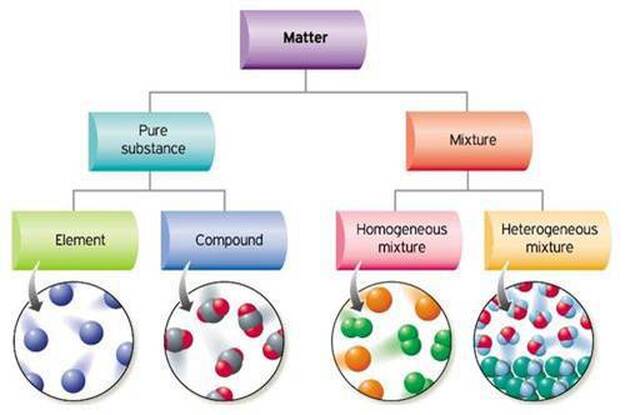 Source: sciencesfp.com
Source: sciencesfp.com
To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. Therefore water is a compound. Properties of a compound. It has two elements hydrogen and oxygen combined in a fixed ratio. A pure substance basically composed of two or more elements and chemically combined in a fixed proportion is called a compound.
Source: docs.google.com
A compound is not a pure substance because it is not an element and only elements are pure substances. To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. A compound is not a pure substance because it is not an element and only elements are pure substances. A substance is one of two forms of matter the other is a mixture. Properties of a compound.
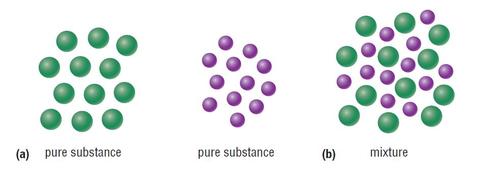 Source: nedssurvivalguide.weebly.com
Source: nedssurvivalguide.weebly.com
Different types of chemical bonds include ionic bonds which are found in salts metallic bonds which are found in metals and covalent bonds which are found in molecular compounds. Matter can be divided into pure substance and mixture then pure substance can be divided into element and compound so from this classification we can say that a compound is a pure substance in pure substance the composition remains fixed an element is formed by only one type of atoms or by atoms which have same atomic number on the other hand a compound is formed by more than one element. A pure substance basically composed of two or more elements and chemically combined in a fixed proportion is called a compound. A compound is a pure substance because its molecule cannot be broken down into simpler particles by physical means. A compound is a pure substance because it consists of two different elements which are pure substances.
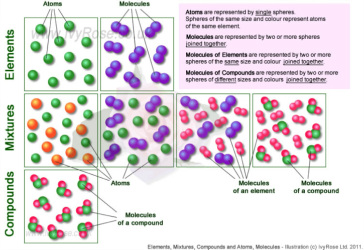 Source: eastmontscience.weebly.com
Source: eastmontscience.weebly.com
To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. Chemical compounds exhibit a unique structure. To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. A compound is a pure substance because it consists of two different elements which are pure substances. A compound is a pure substance because its molecule cannot be broken down into simpler particles by physical means.
 Source: toppr.com
Source: toppr.com
A compound is a pure substance because its molecule cannot be broken down into simpler particles by physical means. Chemical compounds are pure substances which contain two or more chemical elements. It has two elements hydrogen and oxygen combined in a fixed ratio. Therefore water is a compound. To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound.
 Source: quizlet.com
Source: quizlet.com
To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. It has two elements hydrogen and oxygen combined in a fixed ratio. However in many ways the designation pure compound is an oxymoron since all compounds are pure. Therefore water is a compound.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
To understand why all compounds are pure it is important to first understand what constitutes a substance as opposed to a mixture as well as what constitutes a compound. Chemical compounds exhibit a unique structure. However in many ways the designation pure compound is an oxymoron since all compounds are pure. A compound is a pure substance because its molecule cannot be broken down into simpler particles by physical means. A substance is one of two forms of matter the other is a mixture.
Source: uwodesynit782.ygrec.msk.ru
Chemical compounds exhibit a unique structure. However in many ways the designation pure compound is an oxymoron since all compounds are pure. Properties of a compound. A compound is a pure substance because its molecule cannot be broken down into simpler particles by physical means. Chemical compounds are pure substances which contain two or more chemical elements.
 Source: thoughtco.com
Source: thoughtco.com
Chemical compounds are pure substances which contain two or more chemical elements. Therefore water is a compound. Matter can be divided into pure substance and mixture then pure substance can be divided into element and compound so from this classification we can say that a compound is a pure substance in pure substance the composition remains fixed an element is formed by only one type of atoms or by atoms which have same atomic number on the other hand a compound is formed by more than one element. However in many ways the designation pure compound is an oxymoron since all compounds are pure. Properties of a compound.
 Source: chemistrygod.com
Source: chemistrygod.com
Properties of a compound. Therefore water is a compound. A pure substance basically composed of two or more elements and chemically combined in a fixed proportion is called a compound. It has two elements hydrogen and oxygen combined in a fixed ratio. Chemical compounds are pure substances which contain two or more chemical elements.
 Source: quizlet.com
Source: quizlet.com
Properties of a compound. It has two elements hydrogen and oxygen combined in a fixed ratio. Different types of chemical bonds include ionic bonds which are found in salts metallic bonds which are found in metals and covalent bonds which are found in molecular compounds. They are held together in a specific pattern by chemical bonds. A compound is a pure substance because it consists of two different elements which are pure substances.
 Source: diffen.com
Source: diffen.com
Chemical compounds exhibit a unique structure. A pure substance basically composed of two or more elements and chemically combined in a fixed proportion is called a compound. Therefore water is a compound. Properties of a compound. A compound is not a pure substance because it is not an element and only elements are pure substances.
Source: docs.google.com
A pure substance basically composed of two or more elements and chemically combined in a fixed proportion is called a compound. A compound is a pure substance because its molecule cannot be broken down into simpler particles by physical means. A substance is one of two forms of matter the other is a mixture. Therefore water is a compound. They are held together in a specific pattern by chemical bonds.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is a compound a pure substance by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







